User Tools
Table of Contents
About the Bioimaging Platform
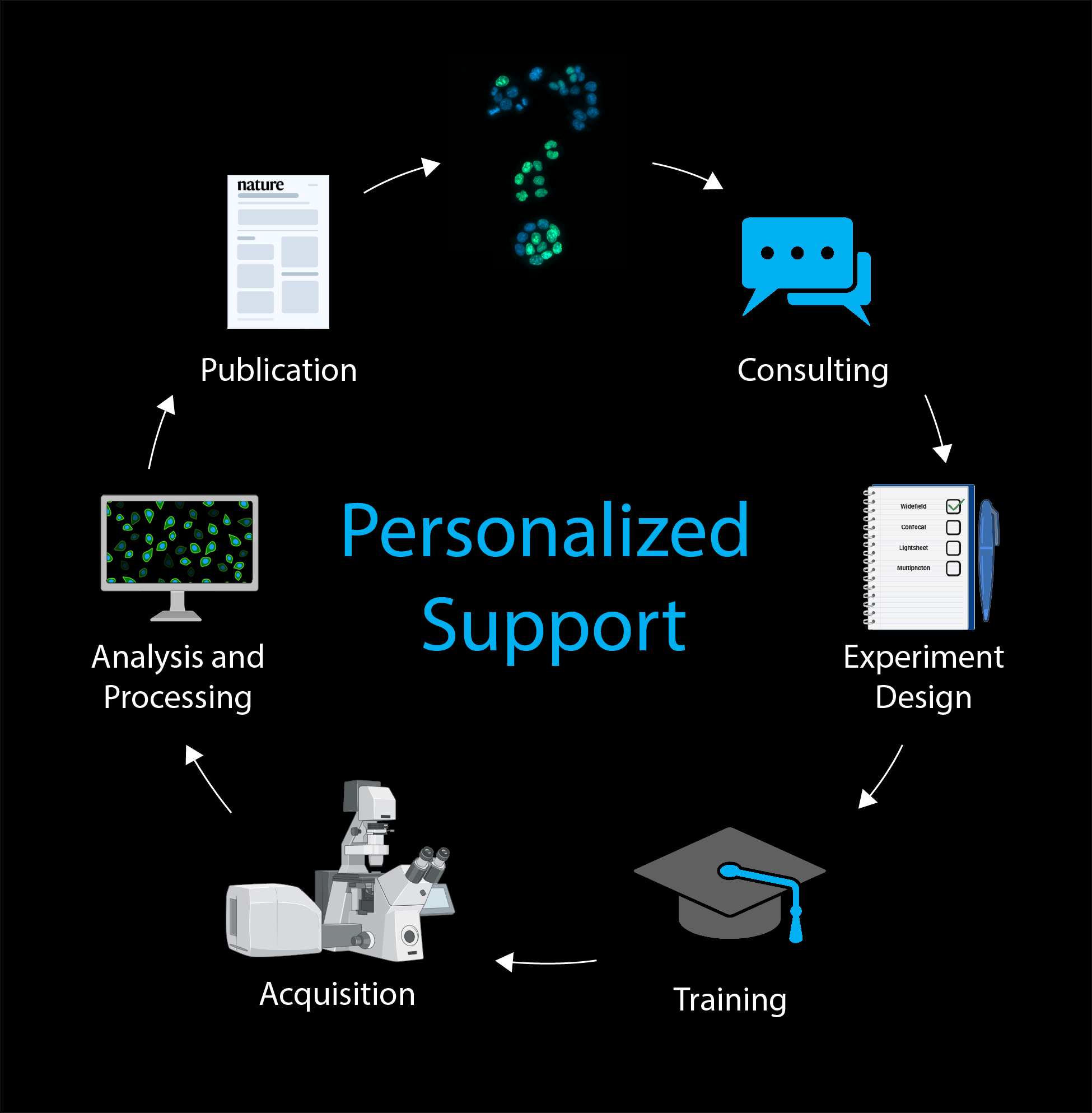
The Bioimaging Platform offers state-of-the-art personalized support in all steps of research done with Light Microscopy at GIMM, housing 29 systems including point-scanning confocal, spinning disk confocal, multiphoton, light sheet, widefield, slide scanning, and advanced image analysis workstations.
From the very first question to the moment of discovery and publication, we sit with researchers to advise, teach, train, and assist in image acquisition, analysis and processing, empowering scientists with cutting-edge technology and expertise.
In addition to daily hands-on training of users, we organize regular courses and workshops in basic and advanced light microscopy techniques and bioimage analysis.
We are a node of Portuguese Platform of Bioimaging (PPBI), a nationwide consortium of imaging facilities which integrates the European research infrastructure Euro-BioImaging ERIC. We are also a ZEISS labs@location Partner, joining a community of advanced microscopy facilities providing in-depth knowledge, dedicated services, and scientific support to ZEISS customers.
How to Acknowledge us

If you used our services, please acknowledge us in publications or other formal presentations. This is one of our key performance indicators and very important for future funding. You are welcome to use the following examples:
Materials and Methods
“Microscopy experiments were performed at the Bioimaging Platform of the Gulbenkian Institute for Molecular Medicine.”
Acknowledgements
“We thank the technical support of the Bioimaging Platform of the Gulbenkian Institute for Molecular Medicine, funded by PPBI-POCI-01-0145-FEDER-022122.”
Co-authorship
Assistance provided above the technical or routine level, where the Platform staff provides scientific input and expertise in experimental set-up, acquisition, analysis or writing, should be recognized through co-authorship on resulting publications. Please check this Global Bioimaging poster for co-authorship guidelines and talk to us, we are happy to discuss possible co-authorship situations.
Thank you!

Services
The Bioimaging Platform provides training and assistance in planning optical microscopy-oriented projects, choosing materials and equipment, preparing samples, acquiring data, analyzing and processing acquired images, presenting and publishing results.
 The Platform houses 29 microscopy systems, including five inverted laser point scanning confocal microscopes with motorized stage, piezo and super-resolution detector prepared for live cell imaging [3x ZEISS LSM 980 with Airyscan 2 (one of them with a 32-channel spectral detector), ZEISS LSM 900 with Airyscan 2, ZEISS LSM 880 with Airyscan), and one fully automated inverted widefield and point scanning confocal microscope optimized for high-throughput live imaging in well-plates that integrates with an advanced robotic loader, automated liquid handler, and incubator to enable high-throughput, fully automated workflows for live-cell imaging (ZEISS Celldiscoverer 7 with LSM 900)], six inverted spinning disk confocal microscopes with motorized stage, piezo and EM-CCD/sCMOS cameras prepared for live cell imaging [3x 3i Marianas SDC (one of them with TIRF and in a BSL2 room), Andor Dragonfly SDC, Leica Spinning Disk Confocal, and ZEISS Cell Observer SD], three light sheet microscopes prepared for water and clearing media immersion imaging (2x ZEISS Lightsheet Z.1, and Miltenyi UltraMicroscope Blaze), one multi-photon microscope (Prairie Multiphoton), seven fully motorized inverted widefield microscopes with cooled CCD/CMOS cameras (Agilent Cytation 5 multimode reader with incubation, Deltavision OMX-SIM super-resolution with incubation and SIM-TIRF, Nikon Eclipse Ti with stage top incubation, Nikon HTM-HCS with incubation and a Ionoptix calcium and contractility measurement system, ZEISS Cell Observer with a large cage incubator and an Akoya PhenoCycler for spatial phenotyping, ZEISS Imager with Apotome 2 with 8 slide carrier, and ZEISS Axiovert 200M), one automated slide scanner for fluorescence and brightfield scanning up to 100 slides (ZEISS Axioscan 7), two fluorescence stereo microscopes with CCD/CMOS cameras (ZEISS Axio Zoom.V16, and ZEISS Stereo Lumar.V12) and other imaging resources such as a brightfield microscope (Leica DM2500) and a bioluminescence/fluorescence imaging system (IVIS Lumina). There are also high spec workstations for image analysis and processing (Amira, Chronos, Huygens, Imaris, Z2, Z3, and ZEISS LSM 980-32 Workstations).
The Platform houses 29 microscopy systems, including five inverted laser point scanning confocal microscopes with motorized stage, piezo and super-resolution detector prepared for live cell imaging [3x ZEISS LSM 980 with Airyscan 2 (one of them with a 32-channel spectral detector), ZEISS LSM 900 with Airyscan 2, ZEISS LSM 880 with Airyscan), and one fully automated inverted widefield and point scanning confocal microscope optimized for high-throughput live imaging in well-plates that integrates with an advanced robotic loader, automated liquid handler, and incubator to enable high-throughput, fully automated workflows for live-cell imaging (ZEISS Celldiscoverer 7 with LSM 900)], six inverted spinning disk confocal microscopes with motorized stage, piezo and EM-CCD/sCMOS cameras prepared for live cell imaging [3x 3i Marianas SDC (one of them with TIRF and in a BSL2 room), Andor Dragonfly SDC, Leica Spinning Disk Confocal, and ZEISS Cell Observer SD], three light sheet microscopes prepared for water and clearing media immersion imaging (2x ZEISS Lightsheet Z.1, and Miltenyi UltraMicroscope Blaze), one multi-photon microscope (Prairie Multiphoton), seven fully motorized inverted widefield microscopes with cooled CCD/CMOS cameras (Agilent Cytation 5 multimode reader with incubation, Deltavision OMX-SIM super-resolution with incubation and SIM-TIRF, Nikon Eclipse Ti with stage top incubation, Nikon HTM-HCS with incubation and a Ionoptix calcium and contractility measurement system, ZEISS Cell Observer with a large cage incubator and an Akoya PhenoCycler for spatial phenotyping, ZEISS Imager with Apotome 2 with 8 slide carrier, and ZEISS Axiovert 200M), one automated slide scanner for fluorescence and brightfield scanning up to 100 slides (ZEISS Axioscan 7), two fluorescence stereo microscopes with CCD/CMOS cameras (ZEISS Axio Zoom.V16, and ZEISS Stereo Lumar.V12) and other imaging resources such as a brightfield microscope (Leica DM2500) and a bioluminescence/fluorescence imaging system (IVIS Lumina). There are also high spec workstations for image analysis and processing (Amira, Chronos, Huygens, Imaris, Z2, Z3, and ZEISS LSM 980-32 Workstations).
The Bioimaging systems are available on the online booking system for trained researchers 7 days a week and 24 hours per day. User training in each particular system is mandatory, even for researchers with previous microscopy experience.
Prices
We charge according to booked time in each microscopy system. Online booking is mandatory for each usage session and cancellations are allowed if they are made prior to reserved time. Users that do not show up at their booked sessions will be charged in full.
How to use the service
All new users must contact the Bioimaging Platform for training in each specific system. This also applies to researchers with previous experience in microscopy. Only trained users are allowed to book usage sessions on a particular microscope.
Contacts
GIMM Bioimaging Platform
bioimaging@gimm.pt

GIMM Lisbon Site
Room P3-A-5
Edifício Egas Moniz
Avenida Professor Egas Moniz
1649-028 Lisboa, Portugal
☎ 47305

GIMM Oeiras Site
Bartolomeu Dias Wing
Rua da Quinta Grande 6
2780-156 Oeiras, Portugal
☎ 214464533
☎ 4533
Hours
9:00 - 17:30 (with a 1h break for lunch) for Training, Assistance and Consultations
(except on Wednesdays 9:00 - 12:00 - weekly all-hands team meeting)
24 hours, 7 days a week for Equipment Usage by authorized, trained users (see Usage Policies)





