Study discovers novel functions of viral protein
Kaposi’s sarcoma-associated herpesvirus (KSHV) is one of the seven known viruses that causes cancer in humans and is responsible for Kaposi’s sarcoma, a disease linked to immunosuppression mainly associated with AIDS. There are no specific therapies for these tumors, and prognosis can be very poor. Now, a new study led by Pedro Simas, group leader at Instituto de Medicina Molecular João Lobo Antunes (iMM; Portugal) and Professor at Faculdade de Medicina da Universidade de Lisboa (FMUL) and Universidade Católica Portuguesa (UCP) and Kenneth M. Kaye, Professor at Harvard Medical School (HMS; USA), discovered a region of viral protein LANA that is key for viral latency and persistent infection inside human cells. These findings published today in the prestigious journal Proceedings of the National Academy of Sciences of the United States of America (PNAS)*, can potentially be used to develop therapy for KSHV tumors since blocking the function of this LANA region is expected to abolish virus persistence, which would eliminate the cancer cells.
KSHV infects human cells, primarily lymphocytes (a type of white blood cells), and through this infection expresses its own genes in the cell, taking over its growth mechanisms causing it to grow out of control, eventually causing cancer, such as Kaposi’s Sarcoma (KS) or a type of lymphoma termed primary effusion lymphoma. Therefore, understanding how viruses modulate host cell function is central to prospects for potential therapies. Critical to this are the viral proteins expressed during the latent phase of infection, which make the virus persist inside these cells and among them, the latency-associated nuclear antigen – LANA – is a central coordinator of the replication and persistence of viral genomes inside host cells.
“Our research group studies the functions of this viral protein LANA for many years. Now, we have discovered a region of LANA that is key for virus to persist. Of particular interest, this LANA region preferentially interacts with a form of a well-known tumor suppressor protein called p53. The interesting aspect of this discovery is that this region, an acidic domain reader, discerns the presence of a specific post-translation modification, acetylation, an add-on attribute that proteins might acquire while they are being produced by the cell”, explains Pedro Simas, senior author of this study. These modifications determine the structure of a protein therefore its function. Through a series of genetic and biochemical experiments using models of infection, researchers now show that the interaction of this specific “reader” region of LANA with unacetylated p53, or possibly other unacetylated proteins may allow the virus to persist in tumor cells.
The finding that KSHV has evolved a mechanism for this type of protein interaction underscores the physiological importance of post-translational modifications in the regulation of persistence infections”, explains Pedro Simas. “These findings could potentially be used to develop therapy of KSHV tumors since blocking the function of this LANA region is expected to abolish virus persistence, which would kill the cancer cells”, adds senior author Kenneth M. Kaye.
This study was performed at iMM and Brigham and Women’s Hospital and Harvard Medical School and funded by the National Institutes of Health (NIH; USA), Fondation ARC pour la recherche sur le cancer (Fondation ARC) and Fundação para a Ciência e a Tecnologia (FCT; Portugal).
*Franceline Juillard#, Marta Pires de Miranda#, Shijun Li, Aura Franco, André F. Seixas, Bing Liua, Ángel L. Álvarez, Min Tan, Agnieszka Szymula, Kenneth M. Kaye* and J. Pedro Simas*(2020). KSHV LANA acetylation-selective acidic domain reader sequence mediates virus persistence. Proc. Natl. Acad. Sci. U.S.A. DOI: 10.1073/pnas.2004809117
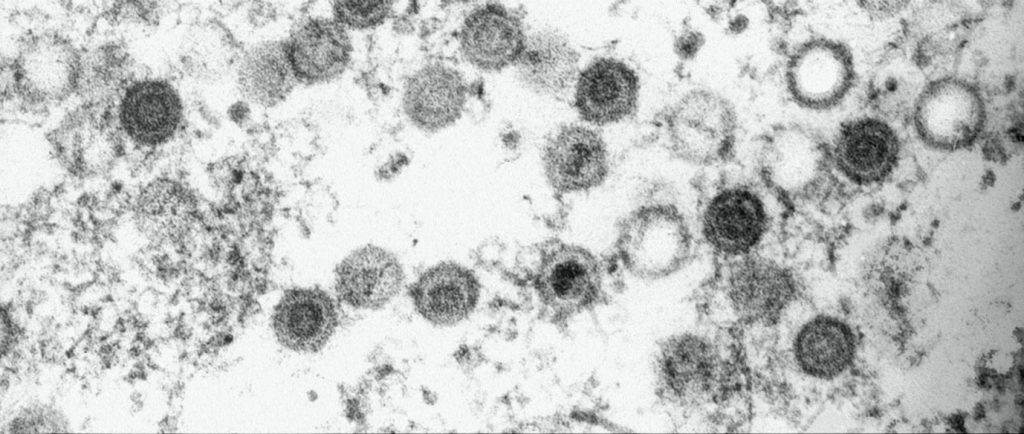
Electron microscopy of MHV-68 virions. MHV-68 is a herpesvirus genetically related to KSHV that is used at iMM to understand the mechanisms of KSHV persistence and carcinogenesis. Credits: Pedro Simas.