Table of Contents
How to prepare samples for Flow Cytometry
How do you get started?
Talk to us about your experiment! If you want to use Flow Cytometry, we can help you design your new experiment. Get in contact with us and we will guide you through the Flow Cytometry Facility, and suggest the best systems for your project. Finally, we will book a training session with you and plan a trial run with your samples.
Here you can find summed up information on the essentials of handling your samples.
For more info check our full document on How to prepare cell samples for Flow Cytometry.
How should you bring your samples?
Obtain a single cell suspension
Your cells must be in a single-cell suspension. Thus, peripheral blood cells or cells that grow in suspension are well suited for analysis by flow cytometry.
Adherent cell lines, solid tissue samples, and tumors require processing into single-cell suspensions before they can be analyzed. The protocol to get your samples into single-cell suspension may involve enzymatic digestion or mechanical dissociation of the tissue. Be careful when performing these protocols, as these may result in the destruction of the antibody epitope or cell damage.
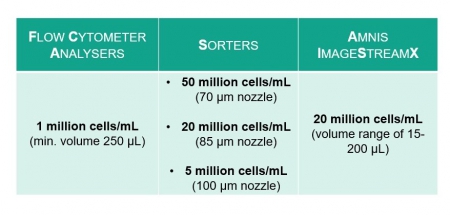
The optimal cell concentration depends on how likely your cells are to clump together in the tube.
These are the recommended concentrations:
In all situations, removing cell clumps, dead cells, and debris is essential to eliminate false positives and obtain results of the highest quality.
After getting a single-cell suspension, you should bring your cells in the right buffer, to avoid reestablishment of cell clumps. A buffer of PBS + 2% FBS/BSA is a good basic buffer.
Please filter all your buffers with a 0.2 µm filter to reduce bioburden and microparticulate contamination.
Adding 25 mM HEPES buffer (pH 7.0) is a good idea as well, as HEPES has better buffering properties at high pressure than PBS does.
You may need to add 1mM EDTA, especially if you have adherent cells, as it helps chelate divalent cations that are often required for the formation of cell aggregates. In addition, if you have a high percentage of dead cells, adding DNase is strongly recommended as it reduces clumping caused by free DNA.
Before analysis or sorting, you should filter your cells through a nylon mesh. For analysis, a 70µm or 100µm mesh (BD Falcon™ cell strainers ref. 352350 and 352360) should work. For sorting, the mesh size should be inferior to the size of the nozzle: 40µm mesh for 70µm nozzle (BD Falcon™ cell strainers ref. 352340) and 70µm mesh for 85µm and 100µm nozzle.
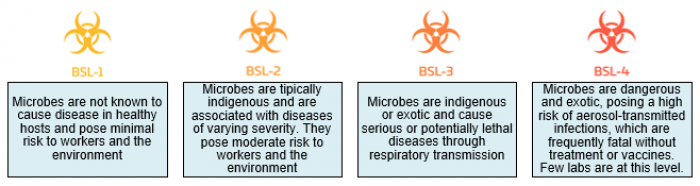
Pay attention to the biosafety level of your samples
Notify us before bringing any cells of human or non-human primate origin to the core facility so we can help you assess the biosafety level of your cells. Most human primary cells and many human cell lines are BSL-2, posing hazards to laboratory staff and the environment.
Only BSL-1 and BSL-2 samples are allowed in the UCF room. BSL-3 and BSL-4 samples are not permitted.
- Analysis of cells by flow cytometry: The analytical cytometers (BD Accuri C6, BD LSRFortessa 2 / X-20, BD FACSymphony A5 SE, CYTEK Aurora and Amnis ImageStream Mark II) are suitable for BSL-1 work only. BSL-2 cells must be fixed before use on these instruments. If your work requires analysis of unfixed BSL-2 cells, this can be done on the BD FACSAria Fusion and BD FACSymphony S6 SE with aerosol containment.
- Sorting of cells by flow cytometry: BSL-2 unfixed cells (e.g. human samples) may only be sorted on the BD FACSAria Fusion and BD FACSymphony S6 SE with aerosol containment. BD FACSAria III can only sort BSL-1 and fixed BSL-2 material.
Many staining protocols include a fixation and/or permeabilization step. However, if yours doesn’t and you need to fix your BSL-2 cells for analysis, we suggest here a quick and easy PFA fixation.
Exclude your dead cells
Even though you keep your cells as happy as possible, some cells can die while handling and performing staining protocols.
Dead cells can affect the analysis by compromising the integrity of the data by non-specifically binding antibodies. That’s why most experiments benefit from adding a viability dye, to exclude dead cells from analysis and sorting. Also this viability staining can help you evaluate and compare the cell apoptosis and necrotic stages between cell conditions.
There are Fixable and Non-Fixable viability dyes. You can choose the right one for your assay depending on the purpose of your analysis and the post-staining protocol that will be performed. .
Perform the desired cell staining
If you are interested in analyzing or isolating specific cell subsets, you can label your cells with fluorochrome-conjugated antibodies or dyes.
- Cell surface markers can be used to define cell subsets based on lineage and developmental stage, as well as function. These surface markers have different forms and functions, including receptors for both soluble and cell-bound ligands, ion channels, glycoproteins, phospholipids, and more.
- Intracellular flow cytometry can be used to analyze a variety of intracellular molecules including cytokines, inflammatory mediators, transcription factors and phosphoproteins. It can provide you rich information about the function and signaling responses of specific cell subsets.
- DNA staining can be used for cell cycle analysis.
While staining of surface markers can be performed in live/unfixed cells, intracellular staining requires cell fixation and permeabilization before staining. Such fixation/permeabilization treatment allows the antibodies against intracellular antigens to cross the plasma membrane to stain intracellularly, while maintaining the morphological characteristic of cells.
Check here our general protocols for cell staining and more detailed information.
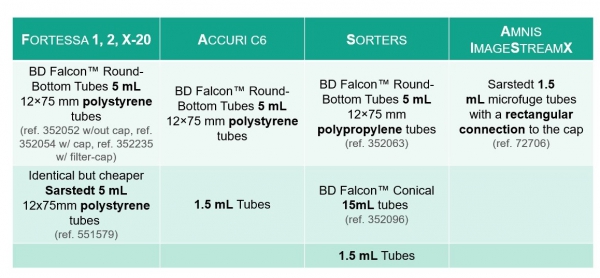
Choose the right tubes
Which controls do you need?
Every experiment needs controls.
Bring unlabeled cells as a negative control. This sample will help you selecting the right cytometer settings for your acquisition. In addition, it will assist you in the analysis, while doing the gating strategy.
If you are using more than one color, you need single-color compensation controls. These will work to evaluate the spectral overlap between all the colors you have. If there is spillover between some colors, compensation will be performed.
For more detailed information about single colors preparation, check out our general staining protocols and some beads you can use for this propose.
For sorting, which collection devices can you use?
- Collect up to four different populations simultaneously into tubes of your choice, 1.5 mL, 5 mL tubes, or up to two populations into 15 mL tubes.
- Sort into any kind of plate. 6-well,12-well, 24-well,36-well, 96-well, 384-well, PCR plate, etc.
The most recommended collection buffer is your cell culture medium with 10% FBS or some other serum. Collection tubes should be about 1/3 full of collection media.
We try to keep everything as clean as possible, but our sorters are not in cell culture hoods, so if you want to culture your sorted cells it’s a good idea to add antibiotics as Pen-strep or gentamycin, and antifungal agents to the collection media. You can also sort directly into lysis buffer (for example, buffer that contains Trizol).
Can you determine the ideal sort conditions?
We can adjust the temperature of the sorters to 4 ºC, 20 ºC, 37 ºC, 42 ºC or at room temperature, and we can keep your sorted cells on ice, if that is what you need.
We need to use the adequate nozzle for your experiment.
Our sorters have the following options:
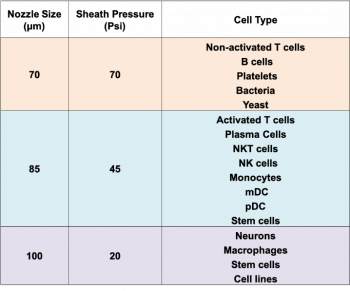
- 70 µm nozzle / 70 psi
- 85 µm nozzle / 45 psi
- 100 µm nozzle / 20 psi
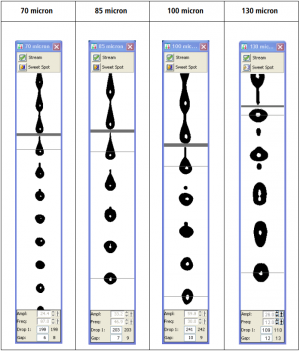 The nozzle selection depends on the size and sensibility of your cells.
The nozzle selection depends on the size and sensibility of your cells.
The most important thing to consider: cells that are fragile or easily stressed (e.g. cultured cells) need a lower pressure sort (100 µm nozzle), independently of their size.
The BD FACSAria sorts faster with a smaller nozzle at higher pressure - 70 µm nozzle. With this nozzle, you can sort up to 20,000 events/second. This nozzle is a great choice for small cells that are happy in single-cell suspension (e.g., blood and bone marrow).
Your cells should be no more than one-third of the size of the nozzle. Therefore, if you have larger cells, they need a larger nozzle size (85 µm or 100 µm nozzle). With these nozzles, sorts are subjected to lower pressures: with the 85 µm nozzle, you can sort at 10,000 events/second, and with the 100 µm nozzle you can sort up to 7,000 events/second.
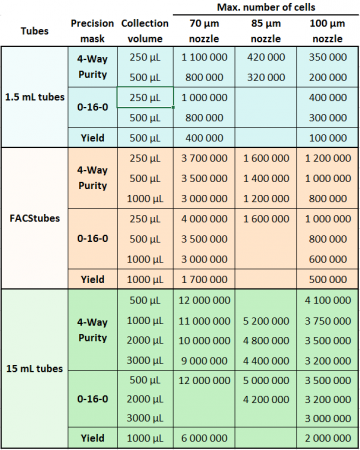
We normally use PBS (0.9% NaCl) in our sorters as sheath fluid. Each individual cell is sorted inside a tiny drop of this sheath fluid. If you are using the 70 µm nozzle (high pressure, faster sorting), the drops are small and you will get approximately 1 ml of sheath fluid with every 1 million sorted cells.
If you are using the 100um nozzle (low pressure, slower sorting), with bigger drops, you will get approximately 3 ml of sheath fluid with every 1 million sorted cells.
What should you bring with you?
| Sorting checklist | Analysis checklist |
|---|---|
| Unstained sample | Unstained sample |
| Single color controls | Single color controls |
| Samples (on ice if appropriate) | Samples (on ice if appropriate) |
| Gating Strategy | Gating Strategy |
| Collection tubes with 1/3 of medium | DIVA password |
| Extra media/PBS for diluting samples | Extra media/PBS for diluting samples |