User Tools
Table of Contents

|
Location: [Oeiras] Room 0B03 |
Microscope overview
 The Zeiss Lightsheet Z.1 is a light sheet fluorescence microscope able to image optical sections of large samples at subcellular resolution and very fast rates, with almost no phototoxicity or bleaching. It splits fluorescence excitation and detection into two separate light paths, with the axis of illumination being perpendicular to the detection axis.
Since only a single thin section of the sample is illuminated by the light sheet, optical sectioning is achieved without a pinhole or image processing deconvolution. Light from the in-focus plane is collected by a sCMOS camera rather than pixel by pixel as in a point scanning laser confocal. This allows you to collect images faster and with less excitation light than you would with many other optical-sectioning microscopy techniques. This system features two objectives with fields of view around 1 mm or 500 µm and resolutions between 0.15 µm and 0.3 µm, equipped with lasers spanning blue to far-red wavelengths (445 nm, 488 nm, 561 nm, and 638 nm) and appropriate fluorescence emission filters. Designed for live imaging, samples are embedded in agarose and preferably loaded into glass or plastic capillaries that provide heating and cooling to maintain physiological conditions, making the system ideal for observing live cells, embryos, Drosophila, and zebrafish. It is important to note that this Z.1 is optimized for live samples and does not include a kit for cleared samples; for such applications, other microscopes are recommended. Users should consult with facility staff to determine the best technique for their specific research needs.
The Zeiss Lightsheet Z.1 is a light sheet fluorescence microscope able to image optical sections of large samples at subcellular resolution and very fast rates, with almost no phototoxicity or bleaching. It splits fluorescence excitation and detection into two separate light paths, with the axis of illumination being perpendicular to the detection axis.
Since only a single thin section of the sample is illuminated by the light sheet, optical sectioning is achieved without a pinhole or image processing deconvolution. Light from the in-focus plane is collected by a sCMOS camera rather than pixel by pixel as in a point scanning laser confocal. This allows you to collect images faster and with less excitation light than you would with many other optical-sectioning microscopy techniques. This system features two objectives with fields of view around 1 mm or 500 µm and resolutions between 0.15 µm and 0.3 µm, equipped with lasers spanning blue to far-red wavelengths (445 nm, 488 nm, 561 nm, and 638 nm) and appropriate fluorescence emission filters. Designed for live imaging, samples are embedded in agarose and preferably loaded into glass or plastic capillaries that provide heating and cooling to maintain physiological conditions, making the system ideal for observing live cells, embryos, Drosophila, and zebrafish. It is important to note that this Z.1 is optimized for live samples and does not include a kit for cleared samples; for such applications, other microscopes are recommended. Users should consult with facility staff to determine the best technique for their specific research needs.
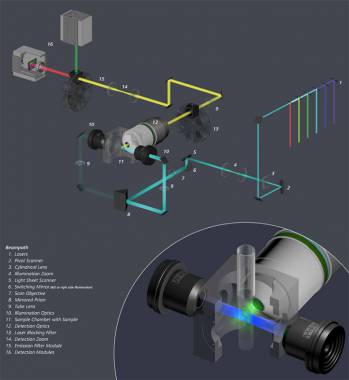
![]() Click on the image on the right to see the system beam path in higher detail
Click on the image on the right to see the system beam path in higher detail
![]() Data files older than 1 month will be automatically deleted on this system, please copy your data to the file server using the desktop link.
Data files older than 1 month will be automatically deleted on this system, please copy your data to the file server using the desktop link.
Additional information for sample preparation
- Sample Size: up to 1 x 1 x 2 cm
Tutorials for Image Analysis with Arivis
System components
LASERs
| LASER | Wavelength | Maximum Power |
|---|---|---|
| Blue-Violet | 445 nm | 20 mW |
| Blue | 488 nm | 50 mW |
| Yellow-Green | 561 nm | 50 mW |
| Red | 638 nm | 75 mW |
Objectives (Illumination)
| Magnification | Model | NA |
|---|---|---|
| 10x | Lightsheet Z.1 10x | 0.20 |
Objectives (Detection)
| Magnification | Model | Immersion | NA | WD (mm) | Reference |
|---|---|---|---|---|---|
| 20x | W Plan-Apochromat DIC 75mm | Water | 1.00 | 2.4 | 421452-9700-000 |
| 40x | W Plan-Apochromat DIC M27 | Water | 1.00 | 2.5 | 421462-9900-799 |
Filtersets
In each experiment you can only use Filter Turret A (installed by default) or B. Please let us know that you want to use filter turret B so that we can install and configure it in advance.
Filter Turret A (default)
| Select Label | Camera #1 Filter | Camera #2 Filter | Fluorophores (Cam #1 : Cam #2) |
|---|---|---|---|
| LP 510 | 460-500 nm | > 585 nm | CFP, mCherry : RFP |
| LP 560 | 505-545 nm | 565-615 nm | GFP, mCherry : RFP |
| LP 560 (2) | 505-545 nm | > 585 nm | GFP, mCherry : RFP |
| LP 560 (3) | 505-545 nm | > 660 nm | GFP, mPlum : mStrawberry, tdTomato |
Filter Turret B
| Select Label | Camera #1 Filter | Camera #2 Filter | Fluorophores (Cam #1 : Cam #2) |
|---|---|---|---|
| LP 510 | 460-500 nm | > 660 nm | CFP, YFP : Cy5 |
| LP 580 | 525-565 nm | > 585 nm | YFP, mCherry : mPlum |
Turn On Procedures
- Turn the system, incubation and PC, in this order.
- If the computer is already turned on, restart it. Start the system and incubator if you are using either CO2, humidity or/and Peltier modules. Check the humidifier bottle, and replace water if necessary.
- Start the ZEN software (icon in Windows desktop), select the “start system” with the “boot status” option on, and confirm that no errors show up.
If you do not start the incubator system, you may receive errors relating to the absence of these systems in ZEN software; however the system should be working as expected. If this is not the case, please contact the facility staff.
Turn Off Procedures
- Place the stage in Load Position and remove your sample.
- Close the software and save your files. Warning: DO NOT CONNECT external disks/USB flash drives to the workstation!
- Shut down the computer once you are done transferring the datasets and will not need to access it remotely. Otherwise, ask the facility staff to turn it off later.
- Turn off the system using the control units (PC, System and Incubation).
- Remove the chamber, disassemble it, and rinse all the parts that compose it with distilled water. Leave them to dry in the appropriate box with the lid opened. Warning: If you have been trained and authorized by the staff to handle objectives, make sure to check and store the 2 illumination objectives, and to place the detection objective in the “washer” before cleaning it. You can ask the facility staff to inspect and clean them to prepare for the next user, if you're not comfortable with this procedure.
- Take your belongings (dishes, gloves, syringe, etc…), and leave the desk ready for the next user.
 ZEISS Lightsheet Z.1 Usage Statistics
ZEISS Lightsheet Z.1 Usage Statistics